Microfluidic system separation CTCs method and background introduction
In recent years, cancer diagnosis and treatment technology has made great progress, but cancer is still the main cause of human death. Cancer metastasis is an important factor in the death of cancer patients. At the same time, the metastatic process is relatively complicated, which increases the difficulty of cancer diagnosis and treatment. Therefore, for cancer, early diagnosis, real-time monitoring and accurate prognosis are crucial.
At present, there are many problems with traditional tissue biopsy methods, such as high cost, difficult sampling, and large trauma, and it is difficult to achieve "early screening and early treatment." With the development of biotechnology, molecular technology and nanotechnology, a series of new technologies have been applied to the diagnosis and treatment of cancer. Liquid biopsy is a frontier field of in vitro diagnostics, covering non-invasive sampling, detecting circulating tumor biomarkers (CTCs, Exosomes, and cNAs) from tumors or metastases released into the blood, providing a new way to monitor tumor dynamics and individualized treatment , has attracted people's attention (Figure 1).

Figure 1: Characteristics of Cancer Formation (A), Metastasis (B), and Circulating Tumor Markers (C)
è´° Microfluidics Application Analysis Liquid Biopsy Background Introduction
1 Microfluidics Technology Overview
Microfluidic technology is a technical means of processing or manipulating liquids at the micron size level. The mixer, actuators, reactors, separators, sensors, etc. are integrated to optimize the detection process. It involves many disciplines such as electronics, mechanics, chemistry, physics and biology. It has the advantages of high flux, high sensitivity, short sample analysis time, small sample size, and strong controllability. It is widely used in modern analytical chemistry and pharmaceuticals. Science, cell biology, genetics and many other research areas. It is also based on the above advantages, microfluidic technology to open the channel for the detection of circulating biomarker biomarkers in liquid biopsy, and provide new effective means for the early diagnosis and treatment of cancer.
2 Application Classification
1) Detection of circulating tumor cells (CTCs)
Circulating tumor cells (CTCs) are tumor cells that fall off from the edge of the primary tumor or metastasis and enter the peripheral blood circulation of cancer patients. Not only do they carry the entire genome of tumor cells, but they already exist in the peripheral blood circulation early in the pathogenesis of cancer. CTCs are used as important targets for cancer research and important markers for early cancer diagnosis and therapeutic evaluation, enabling real-time non-invasive monitoring of cancer development and treatment effects.
In recent years, the separation and purification technologies of CTCs have been continuously developed. However, due to the heterogeneity and rarity of CTCs (only 1-10 CTCs per 107-109 blood cells), the isolation and purification of CTCs is facing great challenges. Microfluidic technology platform is widely used in the separation and purification of CTCs because of its high integration, flexible design, high versatility and high degree of automation.
At present, the method of separating CTCs based on microfluidic technology can be roughly divided into two categories: label-free technology and immunoaffinity technology (Figure 2). The former is based on the physical properties of the separation of the target cells, such as size, density, shape, deformability and dielectric properties, while the latter mainly utilizes the biochemical characteristics of the target cells, and the protein biomarkers specifically expressed on the cell surface (mainly divided Target cells were isolated for anti-EpCAM positive capture and anti-CD45 negative enrichment (Figure 2).
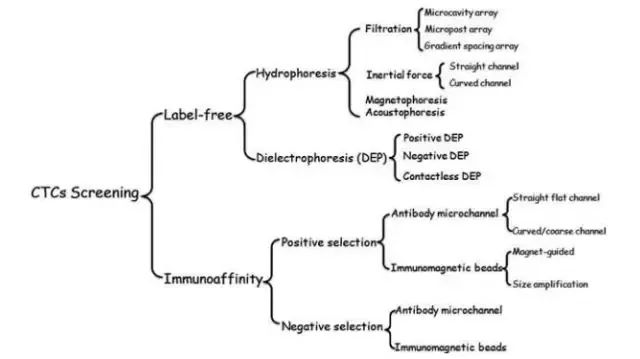
Figure 2: Method of separating CTCs in a microfluidic system
The enrichment of CTCs based on immunomagnetic bead separation is the most common method with high sensitivity and specificity. CellRichTM, a microfluidic-based CTCs detection platform independently developed by Micromated, can efficiently enrich CTCs through the interaction between cancer cell surface antigens and antibodies conjugated to magnetic beads. When the blood flows through the microfluidic device, other blood cells are separated out under the influence of hydrodynamics, and the cells bound to the immunomagnetic beads will be deflected into the separation and enrichment channels by the action of the magnetic field, so as to reach the circulating tumor cells. Separation of the purpose of capture. This method has a higher recovery rate and degree of purification than traditional physical separation methods (Figure 3).

Figure 3: Microfluidic system for separation of CTCs based on immunomagnetic beads
2) Exosome detection
Circular tumor exosomes refer to biologically active vesicle bodies secreted by tumor cells, with a diameter of 20-300 nm. Different sources of exosomes contain their unique lipids, RNA, DNA, and proteins that directly reflect the origin and progression of cells. Exosomes are widely present in human body fluids and have a high abundance (109-1012 exosomes per milliliter of blood). Therefore, the detection and analysis of tumor-derived exosomes can be used as a non-invasive exploration of the origin and progress of tumors, thereby assisting the early diagnosis of tumors and guiding tumor treatment.
In recent years, exosomes as tumor biomarkers have become a hot topic in non-invasive biopsy (liquid biopsy), but secretion of exosomes is a dynamic process that changes in real-time in body fluids and is stable in the course of cancer treatment. Sexual issues. Therefore, the precise separation and analysis of exosomes still faces enormous challenges. Compared with traditional exosome separation technologies such as ultracentrifugation and density gradient centrifugation, microfluidic technology has great advantages in detection automation, integration capability, consumption time, product integrity, purity, and recovery rate. This will facilitate the downstream analysis of exosomes.
At present, the method of separation and purification of exosomes based on microfluidic technology can be divided into two major categories: vesicle size (20-300nm) and surface biomarkers (such as EpCAM, CD9, CD63, CD81), and each has its own characteristics. The former is based on a nanoporous structure, enabling rapid and efficient capture of exosomes. However, because exosomes range in size from nanoscale, they are technically demanding and may be limited by saturation; the latter uses immunoaffinity principles. The established microfluidic systems have antibody-modified microchannels or immunomagnetic beads (Fig. 4) with high throughput and high sensitivity, whereas exosomes also have similar heterogeneity with CTCs and are low for specific biomarkers. The exosome capture efficiency is not high.
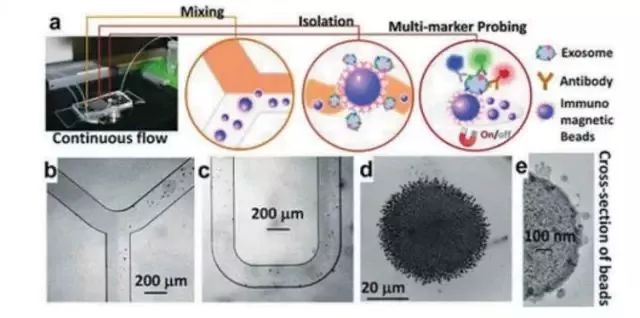
Figure 4: Microfluidic system for analyzing exosomes based on immunoaffinity principle
In addition, although research on exosomes has progressed rapidly, compared with CTCs, there is a lack of mature microfluidic detection systems in the market. In the field of liquid biopsy, there is no extensive and mature CTCs for the study and application of exosomes. Therefore, in order to more accurately quantify and qualitatively analyze tumor-derived exosomes and further explore the physical and biological properties of exosomes, the design of a novel microfluidic system that combines multiple functions into one is essential for the separation and analysis of exosomes. important.
3) cNAs detection
Cyclic Nucleic Acids (cNAs) refer to DNA and RNA fragments found in the blood circulation system. CNAs can be obtained by non-invasive means for analysis of tumor genetic information and epigenetic information. Gene expression profiles are significantly associated with the development and progression of tumors. Therefore, cNAs are important biomarkers for liquid biopsy and can be used for the early diagnosis and therapeutic evaluation of cancer.
Given the immature detection technology, the clinical application of tumor markers cNAs is limited. Traditional methods for extracting and analyzing nucleic acids, such as electrophoresis and fluorescent dyeing, have limitations, are labor-intensive and costly, and step-by-step operations can easily lead to sample loss. The microfluidic system incorporates a variety of technologies. A device can perform multiple operations in sequence, such as sample preparation, reaction, separation, purification, and analysis, reducing sample loss and increasing sample purity.
Therefore, the new microfluidic system can be used as an important means for scientists to study circulating biomarkers, and it is also the future direction for the detection of tumor biomarkers. However, the microfluidic systems currently used for cNAs detection still have problems to be solved: a) Routine extraction of nucleic acids is done step-by-step, the integrity of nucleic acid fragments cannot be guaranteed, analysis errors are easily caused, and development needs to be gentle. A simple and quick method for extracting nucleic acids enables full use of the powerful functions of gene mapping and molecular sequencing technologies. b) Traditional microfluidic systems are simply separation systems, cNAs analysis, qPCR, digital PCR, and sequencing platforms, and PCR methods have inherent limitations such as amplification errors, poor reproducibility, and different amplification efficiencies.
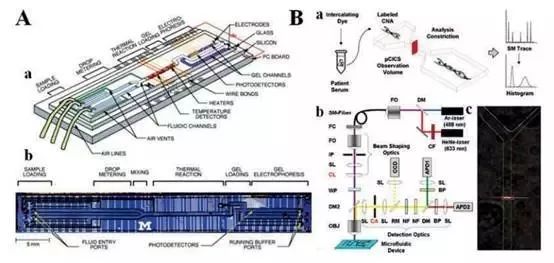
Figure 5: Microfluidic system for analyzing cNAs
In addition, the genetic mutations in different tumors are far more complex than we can imagine. There is a serious tumor heterogeneity, making it more difficult to diagnose and treat cancers by analyzing cNAs. This requires optimizing the performance of existing microfluidic systems and combining gene characteristics to develop more precise, efficient, and highly integrated microfluidic systems to better serve the clinical application of liquid biopsy.
Triple Conclusion
Liquid biopsy as a new non-invasive method of sample acquisition provides a new way for cancer diagnosis and treatment. Microfluidic technology provides an important platform for liquid biopsy because of its advantages of highly integrated and timely handling, and attracts great attention from the scientific community and the biotechnology industry.
At the same time, tumor liquid biopsy still faces numerous challenges in the application of clinical cancer diagnosis and treatment:
a) The three tumor biomarkers are rare and their tumor heterogeneity exists, making isolation and purification very difficult. Overcoming this challenge requires the development of a microfluidic platform that makes full use of the physical and biological properties of tumor biomarkers.
b) High-precision, fully-automated systems based on microfluidic separation and purification of tumor biomarkers are mostly in the scientific research stage and have not been widely used clinically.
c) The relationship between the circulating tumor biomarkers and the microfluidic platform needs to be improved. To further promote the development of a microfluidic system with high sensitivity and good reproducibility, a large number of clinical trials are needed in many cancer patients.
d) Our understanding of tumor biology is still in its infancy. With the continuous in-depth study of the role of various circulating tumor biomarkers in tumor formation and development, a more powerful and more effective commercial microfluidic system will emerge.
It is believed that in the future, microfluidic technology will play an increasingly important role in the field of tumor liquid biopsy and individualized diagnosis and treatment.
Mini Inverter,Mini Frequency Inverter,Compact Frequency Inverter,Ac Frequency Drive For Motor
Zhejiang Kaimin Electric Co., Ltd. , https://www.ckmineinverter.com